July 4th - STITCH WORKSHOP
Workshop
Modernizing Clinical Trials
S.T.I.T.C.H in collaborazione con Fondazione Smith Kline, Verona ed AlfaTi, Milano
INTRODUZIONE
Sebastiano Filetti
RELATORI
Digital Health per Smart Health Giuseppe Recchia, Fondazione Smith Kline, Verona
Although health interventions are only one of the determinants of health, preventing and treating diseases is a fundamental aspect of contributing to healthy ageing and the search for new and more effective interventions is a necessary condition for maintaining their effectiveness over time and responding to the emerging health needs of an ageing population. The need to increase the effectiveness and breadth of health interventions while containing their costs is both a challenge and a major driver for the digital transformation of medicine and health and the development of Digital Health.
Digital Health encompasses both aspects of health managed by health care (telemedicine, information systems and electronic health records) and aspects related to the management of individual health through applications on mobile devices (mApps), sensors and other technologies for personal use, able to generate data and information that are not always integrated with health information systems today. In both cases, the ultimate goal is to make health "smarter" (Smart Health), i.e. more efficient health services and absolute gain in health. Within the concept of Digital Health, an area more recently defined is represented by Digital Medicine, i.e. DHT with the specific purpose of (a) measuring biological or functional parameters (b) intervene with the aim of improving the health status. Among the areas of major interest of Digital Medicine, there is Digital Therapy. What qualifies it as a therapy and therefore different from other digital health technologies is therefore the documentation of effectiveness and tolerability generated through RCTs for the generation of clinical data.
Intelligenza Artificiale e Sperimentazione Clinica Massimo Beccaria, AlfaTI, Milano
The world is evolving in every sector thanks to the great driver of technologies that invade our lives, every day more and more. The world of research, as already happened with those of logistics, mechanics, and telecommunications, is beginning a metamorphosis that will change its current connotations and project it into a future of challenges and new achievements, thanks to the opportunities introduced by digital technologies, such as artificial intelligence.
The planned intervention is intended to be a starting point for reflection on how this scenario, which is only now beginning to be glimpsed, is shaping the current world and how it will irrevocably determine that of the immediate future, what obstacles the Italian system, on the other hand, enmeshes with respect to international dynamics, which is capable of generating talent in this field but is often unable to exploit them.The A.I. applied to clinical trials is already able to shorten times and optimize processes more and more, but like any technology must be managed and standardized, consistent with the objectives set.
Today, therefore, there are difficult challenges that see us once again involved in having to find the right compromises between systems in rapid evolution, exciting expectations and organizational and cultural limits...
In silico trials: un modello per le malattie rare? Enrico Tronci, Dipartimento di Informatica
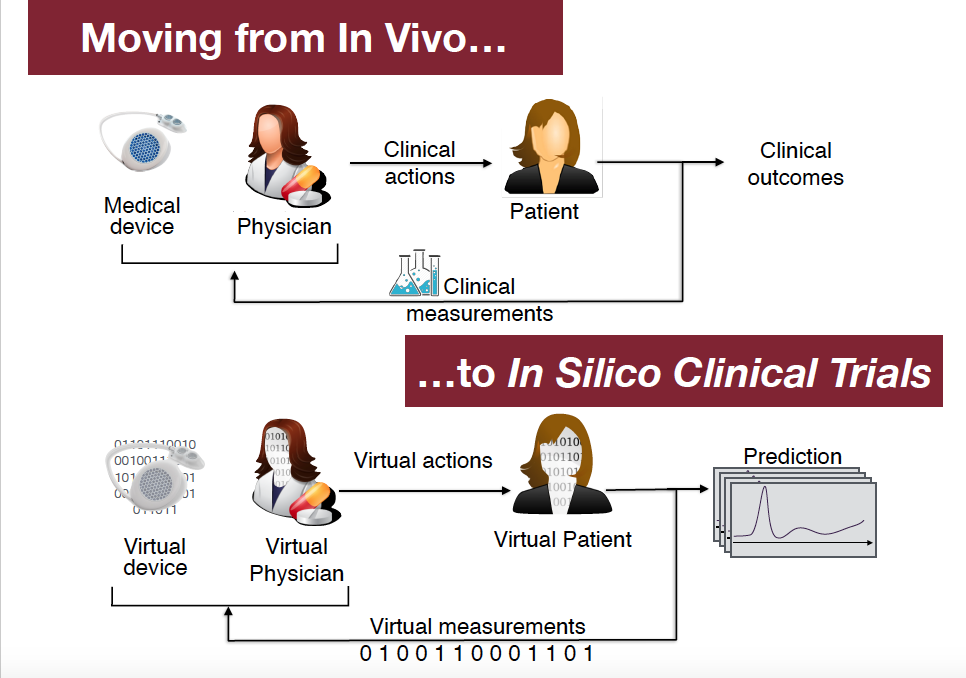
In silico clinical trials (ISCT) aim at replacing the first stages of in vivo clinical trials with computer simulations. This enables safety and efficacy assessment of drugs when there are not enough patients for in vivo clinical trials (e.g., rare diseases) and, more in general, holds the promise of decreasing time and cost of safety and efficacy assessment for drugs and biomedical devices, even when patients may be available.
Through computational models (virtual patients) ISCT predicts, through simulation, the effect of a drug or biomedical device on (real) patients. Basically, ISCT rests on Physiologically based Pharmacokinetic/Pharmacodynamic (PBPK/PD) models as well as on software tools supporting their construction and automatic analysis. In this talk, through examples, we will outline some ISCT methods, software tools and open problems.
giovedì 4 luglio 2019, ore 15.00 - 17.00 Aula A - Edificio di Anatomia umana
via Alfonso Borelli 50, Roma